Retatrutide has become a major focus in modern metabolic and obesity research. Developed by Eli Lilly, this triple agonist peptide activates three key hormone receptors — GLP-1, GIP, and glucagon receptors — influencing glucose metabolism, energy expenditure, and fat oxidation. Understanding how retatrutide dosing works is critical for interpreting laboratory and clinical trials exploring its potential role in weight management and diabetes-related research.
Understanding How Retatrutide Works
Retatrutide works by mimicking incretin hormones that help regulate appetite, blood sugar, and fat metabolism. Its interaction with three hormone receptors enhances insulin secretion, promotes calorie burning, and may drive significant weight loss in early clinical trials. As a triple agonist, retatrutide activates GLP-1, GIP, and glucagon pathways — producing greater metabolic effects than single-acting GLP-1 drugs.
According to The New England Journal of Medicine, early clinical trials show participants achieving up to 24% body weight reduction at the highest studied dose. These results indicate a dose-dependent response where the body adjusts gradually over a structured titration schedule, typically expressed in mg per week.
Typical Retatrutide Dosage and Dosing Strategy
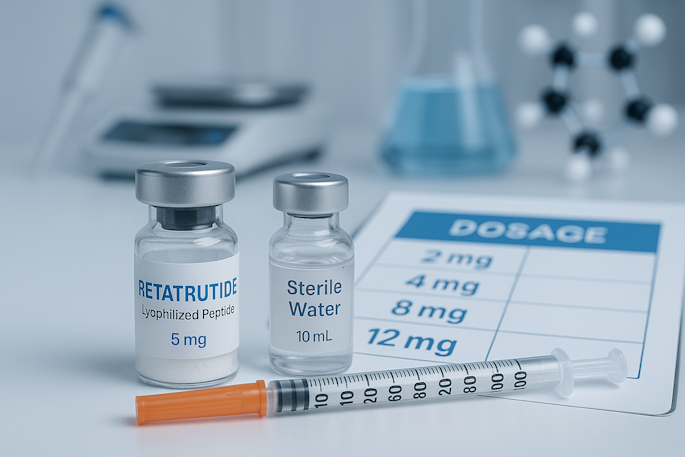
Clinical studies and laboratory models commonly use a once weekly subcutaneous injection approach. The starting dose is conservative, allowing the body to adapt before higher concentrations are introduced. A typical starting dose is 2 mg per week, with gradual dose increases every four weeks until an effective dose or maintenance dose is achieved.
Researchers following clinical data often apply the following dosing schedule to model metabolic health and weight loss outcomes:
- Weeks 1–4: 2 mg per week
- Weeks 5–8: 4 mg per week
- Weeks 9–12: 8 mg per week
- Weeks 13–16: 12 mg per week (maintenance dose)
This titration schedule helps minimize gastrointestinal side effects while maintaining consistent metabolic signaling. The gradual dose escalation mirrors early clinical trials, where participants tolerated lower doses initially before progressing to the highest doses.
Prefer a gentler titration? Read our Retatrutide Microdosing Guide.
Weekly Dosage Chart (mg per Week)
The following retatrutide dosage chart summarizes the most common dosing strategy explored in research and published clinical studies:
| Weeks | MG per Week | Purpose |
|---|---|---|
| Weeks 1–4 | 2 mg weekly | Initial titration / body adjustment |
| Weeks 5–8 | 4 mg weekly | Further receptor activation / body adapts |
| Weeks 9–12 | 8 mg weekly | Mid-phase stabilization |
| Weeks 13+ | 12 mg weekly | Maintenance dose for weight loss progress |
These different doses provide insight into how the body responds as dosage increases. Clinical trials have reported that most participants tolerated up to 12 mg per week with minimal adverse events when titrated correctly.
Retatrutide Dosing in Clinical Research
Retatrutide dosing in clinical research is carefully structured to evaluate safety, tolerance, and weight loss results. Early phase clinical trials published in the New England Journal of Medicine demonstrated that subjects on 8 mg weekly and 12 mg weekly achieved superior weight loss outcomes compared with other weight loss medications targeting only GLP-1 receptors.
Because retatrutide activates three hormone receptors simultaneously, it triggers more comprehensive metabolic responses, improving blood sugar control and supporting overall metabolic health. Researchers noted better insulin response, lower blood pressure, and enhanced fat metabolism compared to baseline.
As the body reacts and the body adjusts, individual tolerance guides the right dose for ongoing weight loss progress and safe maintenance.
Understanding the Starting and Maintenance Dose
The typical starting dose for retatrutide is 2 mg per week, allowing the body time to adapt to incretin activation. Researchers gradually increase to 4 mg, 8 mg, and 12 mg as tolerance develops. The maintenance dose, generally between 8–12 mg weekly, sustains the compound’s metabolic activity and continued weight loss effects.
Clinical research suggests that dose increases spaced several weeks apart reduce the likelihood of side effects and allow stable hormone receptor activation. For participants with underlying health issues or prior exposure to incretin-based therapies, adjusting dosages may be necessary under the supervision of a healthcare provider.
Clinical Trials and Weight Loss Outcomes
Clinical trials for this peptide have consistently shown substantial weight loss results. In a 2023 randomized trial published in the ClinicalTrials.gov database, participants experienced an average of 17–24% total body weight reduction after 48 weeks of treatment. This significant weight loss was maintained through continued weekly dosing.
Unlike many other weight loss medications, retatrutide targets three incretin pathways, resulting in more robust energy balance control and appetite regulation. Over time, body weight stabilizes near each subject’s goal weight, and weight reduction continues gradually with maintenance dosing.
Factors That Influence the Right Dose
Individual variables such as body weight, metabolic health, and underlying health conditions influence the effective dose of retatrutide. Not everyone responds identically; the body responds differently depending on hormone receptor density, genetic factors, and prior weight-related conditions.
Researchers also monitor blood sugar, blood pressure, and energy levels to evaluate safety and ongoing adaptation. Adjusting dosages within the established mg per week range ensures that clinical outcomes remain consistent with target metabolic parameters.
Side Effects and Safety Profile
Like most incretin agonists, retatrutide can produce mild to moderate gastrointestinal side effects during dose escalation. Common side effects include nausea, bloating, reduced appetite, or mild fatigue as the body adjusts. In rare cases, participants reported constipation or transient dizziness.
Long-term safety data remain under evaluation in ongoing clinical studies. Laboratory handling requires adherence to sterile preparation and once weekly injection standards to maintain sample integrity. Retatrutide remains in early research phases and is not yet FDA approved for consumer use.
Learn proper mixing methods in our How To Reconstitute Retatrutide Guide.
How Retatrutide Compares to Other Weight Loss Medications
Compared to other weight loss medications such as tirzepatide or semaglutide, retatrutide activates an additional glucagon receptor, giving it broader influence over fat metabolism and energy expenditure. Its triple agonist mechanism may translate to higher potential for weight reduction and improved glucose homeostasis, as demonstrated in early clinical trials.
Because this peptide works on three key hormone receptors, researchers expect improved long-term metabolic adaptation and lower risk of plateauing weight loss progress during maintenance dose phases.
Compare against GLP-1 standards with our Retatrutide vs Semaglutide Analysis.
Dosing Strategy and Clinical Implications
A well-structured dosing strategy is essential for achieving predictable weight loss results. The titration schedule — increasing the dose gradually — allows incretin hormones to stabilize appetite and improve glucose dependent insulinotropic polypeptide activity.
The dosing schedule also helps researchers observe how the body reacts across different doses, identifying the most effective dose for sustained weight reduction and long-term metabolic balance. Clinical data show that participants on a once weekly subcutaneous injection regimen experienced continuous improvements in glucose control and weight loss outcomes.
Switching from Tirzepatide? Follow our Transition Guide.
When to Adjust the Dose or Handle a Missed Dose
If a missed dose occurs, researchers or clinicians typically allow for administration within four days, maintaining the weekly rhythm. After that, the dose should resume as scheduled the following week.
Adjusting dosages should be performed cautiously under medical supervision, especially in participants with health issues such as cardiovascular disease or diabetes. Healthcare providers typically review participant progress, body weight trends, and any side effects before making dose increases.
Long-Term Weight Loss and Metabolic Health Benefits
Over multiple months of continuous dosing, participants in clinical trials exhibited improved insulin sensitivity, reduced blood sugar, lower blood pressure, and enhanced metabolic health markers. Fat metabolism efficiency improved as the dosage reached maintenance levels, and total energy expenditure increased.
These findings underscore retatrutide’s potential as a powerful agent for obesity and metabolic research — though not everyone experiences the same pace of body weight reduction or energy balance adaptation.
Frequently Asked Questions
What is the effective dose of retatrutide for weight loss research?
The effective dose varies, but most clinical trials use between 8–12 mg per week as a maintenance dose. These levels maintain stable receptor activation for continued weight loss and metabolic benefits.
Can you drink alcohol while taking retatrutide?
While no direct contraindications are established, alcohol may affect blood sugar and digestion, potentially amplifying side effects during early dose escalation. Researchers in clinical trials advised participants to limit alcohol intake.
What if I miss a weekly injection?
If a once weekly injection is missed, it can usually be administered within four days. Otherwise, wait until the next scheduled dose. The key is maintaining a consistent dosing schedule for reliable results.
Is retatrutide FDA approved?
As of now, retatrutide is not FDA approved. It remains under active clinical evaluation for weight loss and metabolic disorders.
How can I purchase retatrutide for research?
You can purchase retatrutide only from licensed suppliers providing research-grade compounds. Always verify Certificates of Analysis and purity documentation before acquisition.
Check out our main Where To Get Retatrutide page and review Bulk Retatrutide From China Risks.
Conclusion
Retatrutide dosage research shows remarkable promise for future weight management, weight loss, and metabolic applications. Its activation of three key hormone receptors makes it distinct from other weight loss medications and capable of achieving significant weight loss through improved glucose, insulin, and fat metabolism regulation.
While long-term safety data continue to emerge, early clinical trials published in the England Journal of Medicine and other peer-reviewed outlets demonstrate sustained weight reduction, improved metabolic health, and durable blood pressure and blood sugar control.
For now, all dosing information remains strictly for research purposes. Researchers following structured titration schedules — from 2 mg per week up to 12 mg weekly — can better understand how the body adjusts, how the body responds to incretin modulation, and how dose increases affect weight loss outcomes and metabolic stability.