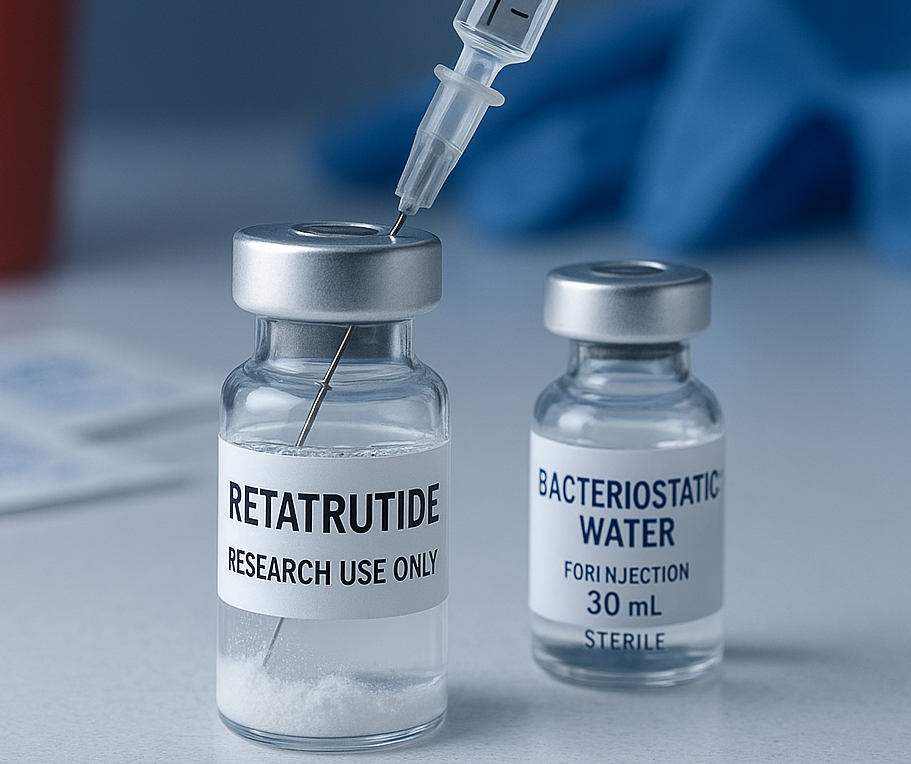
Properly preparing Retatrutide for research use is a critical step in maintaining the peptide’s stability, accuracy, and potency. This triple-agonist compound, which is known for its action on GLP-1, GIP, and glucagon receptors, has become a key focus in metabolic and weight management studies.
To ensure you’re sourcing legitimate product, check our main guide on Where To Get Retatrutide.
In early clinical trials, scientists found that Retatrutide’s effects depend heavily on the quality of the reconstituted solution. Ensuring sterile technique, correct dilution ratios, and precise measurements helps produce consistent results and reliable data during peptide-based research.
Understanding Retatrutide Reconstitution
Reconstitution refers to the process of mixing a lyophilized peptide vial with bacteriostatic water or sterile water to form a usable solution. Retatrutide is typically supplied as a freeze-dried powder in a sealed vial along with other products. This form increases stability during shipping and storage, allowing for consistent potency across multiple doses. The process requires precision, sterile techniques, and awareness of correct volumes and concentrations.
According to a study in the New England Journal of Medicine, Retatrutide’s formulation was designed for once-weekly subcutaneous injection models for patients to evaluate long-term effects on energy balance and metabolism. To replicate proper study conditions, laboratory professionals must handle each peptide vial using aseptic methods and approved laboratory safety practices.
Considering foreign vendors? Read Can You Trust Bulk Retatrutide From China?
Supplies Needed to Prepare Retatrutide
Before beginning the reconstitution process, prepare the following materials to ensure sterility and accuracy:
- Retatrutide lyophilized peptide vial (5 mg, 10 mg, or 12 mg)
- Bacteriostatic water (sterile water with benzyl alcohol to prevent bacterial growth)
- 1 mL or 3 mL insulin syringes with 29–31 gauge needles
- Alcohol swabs and sterile gloves
- Clean laboratory workspace with aseptic protection
- Sharps container for safe disposal
- Labeling tape or printed labels for concentration and date tracking
Every step must be performed in a sterile environment to protect peptide integrity. Vigorous shaking, touching the stopper with bare hands, or improper water use can lead to peptide degradation and inaccurate concentrations, which may result in side effects.
Step-by-Step Guide on Retatrutide Reconstitution
Step 1 – Sanitize and Prepare
Clean your workspace with alcohol and allow surfaces to dry. Put on sterile gloves before handling the vial or water. Remove the protective caps from both the Retatrutide vial and bacteriostatic water vial. Wipe each rubber stopper with an alcohol swab.
Step 2 – Draw Bacteriostatic Water
Using a clean syringe, draw the required volume of bacteriostatic water. Most researchers use 2 mL for a 10 mg vial or 1 mL for a 5 mg vial, depending on the desired concentration. The amount of water added determines the final mg/mL concentration used in research dosing calculations.
Step 3 – Add the Water to the Vial
Insert the syringe needle into the peptide vial at a slight angle and slowly add the water. Let the stream of water run gently down the side of the glass. Avoid vigorous shaking; instead, swirl the vial slowly to dissolve the powder. This approach prevents peptide chain breakage and preserves molecular structure.
Step 4 – Allow the Solution to Dissolve
Let the reconstituted solution rest for several minutes at room temperature. The solution should become clear, with no visible particles or cloudiness, as clarity is important to avoid nausea after administration. If clumping remains, continue gentle swirling. Do not expose the vial to heat, light, or air for extended periods.
Step 5 – Label and Store the Vial
Once fully dissolved, label the vial with the date, concentration (mg/mL), and study number. Store it in a refrigerator between 36°F–46°F (2°C–8°C) to help prevent side effects such as vomiting. Never freeze the reconstituted solution, as freezing can damage peptide structure and reduce activity.
Example Retatrutide Reconstitution Ratios
Below is an example Retatrutide reconstitution chart to help determine appropriate volumes and concentrations, including options such as 4 mg.
| Peptide Vial | Bacteriostatic Water Added | Final Concentration (mg/mL) | Units per mg |
|---|---|---|---|
| 5 mg | 1 mL | 5 mg/mL | 20 units = 1 mg |
| 10 mg | 2 mL | 5 mg/mL | 20 units = 1 mg |
| 12 mg | 2.4 mL | 5 mg/mL | 20 units = 1 mg |
These calculations are based on a 100-unit insulin syringe where 1 mL equals 100 units. Researchers can determine the exact dosage concentration by dividing total mg by total mL of water added. The formula used is:
Concentration (mg/mL) = Total mg ÷ Total mL
Administering and Injection Sites (For Research Models)
When administering Retatrutide in laboratory animal models, the once-weekly injection schedule is typically used to mimic human clinical protocols. Multiple injections should be avoided; instead, one controlled dose is delivered per week. Common injection sites in research studies include the abdomen, thigh, or upper arm regions. Each site should be rotated weekly to avoid tissue irritation.
The peptide solution is usually drawn using a 1 mL syringe and injected subcutaneously. The volume of each weekly dose depends on the experimental protocol and concentration of the reconstituted solution. Always document the total units delivered and store unused solution under refrigeration.
Review our Retatrutide Dosage Chart for weekly administration intervals.
Storage and Stability of the Reconstituted Solution
Once Retatrutide has been reconstituted, store the vial in a refrigerator between 36°F and 46°F (2°C–8°C). The solution typically remains stable for up to four weeks, depending on storage conditions and the sterility of bacteriostatic water used. Always inspect before each use—if the solution appears cloudy or has visible particles, discard immediately.
Proper storage ensures consistent results across weekly dosing studies. Peptides like Retatrutide can lose potency if left at room temperature for extended time periods. To continue maintaining accuracy, label each vial with preparation date and concentration, allowing laboratory staff to track stability over time.
For fine-tuned dosing adjustments, see How To Microdose Retatrutide.
Safety and Handling Considerations
Laboratories should handle Retatrutide as a research-only medicine under established biosafety protocols. Avoid direct skin contact, and dispose of needles in a sharps container. Since this peptide interacts with glucagon receptors and affects metabolic signaling, researchers should follow guidelines for peptide handling as recommended by institutional review boards and published NIH safety standards.
Potential side effects observed during clinical research include mild nausea, vomiting, diarrhea, and constipation. These effects were dose-dependent and generally subsided as the body adapted to weekly dosing schedules. Always record any reactions observed during testing.
Common Mistakes During Reconstitution
- Using tap water instead of bacteriostatic water
- Applying vigorous shaking instead of gentle swirling
- Failing to refrigerate the reconstituted solution
- Not labeling the vial with concentration or date
- Over-diluting or under-diluting the peptide solution
Each of these errors can lead to inaccurate concentration, reduced peptide stability, or contamination. Consistent reconstitution techniques help ensure that each weekly dose delivers the intended results in ongoing research.
Scientific Context and Clinical Relevance
In early clinical trials, Retatrutide demonstrated significant effects on energy expenditure and glucose control when used in weekly dosing schedules. These trials showed that Retatrutide’s activation of GLP-1, GIP, and glucagon receptors can improve metabolic balance, enhance fat oxidation, and support weight management outcomes in study populations.
While these results were promising, Retatrutide remains under research evaluation and is not an FDA-approved medication. Each peptide vial used for reconstitution must be labeled “For Research Use Only,” ensuring it is not administered to patients or individuals outside controlled laboratory studies.
Frequently Asked Questions
How much reconstitution solution for 10 mg semaglutide?
For a 10 mg semaglutide vial, most researchers add 2 mL of bacteriostatic water to achieve a 5 mg/mL concentration based on established protocols. This same method can be applied when you prepare Retatrutide or other GLP-1 related peptides, allowing consistent mg-per-unit calculations in experimental models.
How long should you cycle Retatrutide?
Research schedules vary, but typical models use once-weekly dosing for up to 12–16 weeks depending on study design. This approach allows time to monitor the body’s response and metabolic adaptations while ensuring the reconstituted solution remains within its stability window.
How to reconstitute 5 mg Tirzepatide?
To reconstitute a 5 mg Tirzepatide vial, add 1 mL of bacteriostatic water using gentle swirling techniques. Avoid vigorous shaking, which can damage peptide chains. The resulting solution is 5 mg/mL and suitable for use in laboratory settings that compare multiple GLP-1 receptor agonists like Retatrutide and Tirzepatide.
How to calculate reconstitution?
To calculate your concentration, divide total peptide mg by total mL of water added. For example, adding 2 mL to a 10 mg vial results in 5 mg/mL concentration. This calculation allows you to determine how many units correspond to each mg of peptide in your dosing schedule. Always confirm numbers before administering.
Final Thoughts
Learning how to reconstitute Retatrutide safely and accurately is crucial for high-quality research outcomes. Using bacteriostatic water, avoiding vigorous shaking, and following proper labeling and storage practices ensure that each peptide vial delivers consistent results across time. Each weekly dose should be carefully documented, stored at proper temperature, and disposed of following biosafety standards.
Retatrutide continues to gain attention in metabolic and weight management research for its multi-receptor activity as a promising medicine nd strong early data in clinical trials. As ongoing reviews and published results expand, researchers can expect more refined dosage and stability guidelines to emerge, allowing even greater precision when preparing the reconstituted solution for future experiments.
If transitioning from Tirzepatide, consult our Switching From Tirzepatide to Retatrutide Guide.